It is of utmost significance for the entire human race and its study has lead us to deeper understanding of God's creation. Most of the things we know about Cosmos we know through the study of light.
Today we know more about light largely because the great Albert Einstein paid attention to small details. He was not happy with the nasty anomalies that made the existing scientific theories somewhat ugly. He had a beautiful mind.
For our convenience, I quote here key parts of the wikipedia article summarizing his breakthrough theories and their consequences to modern science instead of paraphrasing it in my own words (my emphasis).
1905 was a good year.
Special theory of relativity
The wave theory was successful in explaining nearly all optical and electromagnetic phenomena, and was a great triumph of nineteenth century physics.
By the late nineteenth century, however, a handful of experimental anomalies remained that could not be explained by or were in direct conflict with the wave theory.
One of these anomalies involved a controversy over the speed of light. The constant speed of light predicted by Maxwell's equations and confirmed by the Michelson-Morley experiment contradicted the mechanical laws of motion that had been unchallenged since the time of Galileo, which stated that all speeds were relative to the speed of the observer.
In 1905, Albert Einstein resolved this paradox by proposing that space and time appeared to be changeable entities, which accounted for the constancy of the speed of light.
Einstein also proposed a previously unknown fundamental equivalence between energy and mass with his famous equation
where E is energy, m is, depending on the context, the rest mass or the relativistic mass, and c is the speed of light in a vacuum.
Particle theory revisited
Another experimental anomaly was the photoelectric effect, by which light striking a metal surface ejected electrons from the surface, causing an electric current to flow across an applied voltage.
Experimental measurements demonstrated that the energy of individual ejected electrons was proportional to the frequency, rather than the intensity, of the light.
Furthermore, below a certain minimum frequency, which depended on the particular metal, no current would flow regardless of the intensity.
These observations appeared to contradict the wave theory, and for years physicists tried in vain to find an explanation.
In 1905, Einstein solved this puzzle as well, this time by resurrecting the particle theory of light to explain the observed effect. Because of the preponderance of evidence in favor of the wave theory, however, Einstein's ideas were met initially with great skepticism among established physicists. But eventually Einstein's explanation of the photoelectric effect would triumph, and it ultimately formed the basis for wave–particle duality and much of quantum mechanics.
Quantum theory
A third anomaly that arose in the late 19th century involved a contradiction between the wave theory of light and measurements of the electromagnetic spectrum emitted by thermal radiators, or so-called black bodies. Physicists struggled with this problem, which later became known as the ultraviolet catastrophe, unsuccessfully for many years.
In 1900, Max Planck developed a new theory of black-body radiation that explained the observed spectrum. Planck's theory was based on the idea that black bodies emit light (and other electromagnetic radiation) only as discrete bundles or packets of energy. These packets were called quanta, and the particle of light was given the name photon, to correspond with other particles being described around this time, such as the electron and proton.
A photon has an energy, E, proportional to its frequency, f, by where h is Planck's constant, λ is the wavelength and c is the speed of light. Likewise, the momentum p of a photon is also proportional to its frequency and inversely proportional to its wavelength: As it originally stood, this theory did not explain the simultaneous wave- and particle-like natures of light, though Planck would later work on theories that did. In 1918, Planck received the Nobel Prize in Physics for his part in the founding of quantum theory.
Wave–particle duality
The modern theory that explains the nature of light includes the notion of wave–particle duality, described by Albert Einstein in the early 1900s, based on his study of the photoelectric effect and Planck's results.
Einstein asserted that the energy of a photon is proportional to its frequency.
More generally, the theory states that everything has both a particle nature and a wave nature, and various experiments can be done to bring out one or the other.
The particle nature is more easily discerned if an object has a large mass, and it was not until a bold proposition by Louis de Broglie in 1924 that the scientific community realised that electrons also exhibited wave–particle duality. The wave nature of electrons was experimentally demonstrated by Davisson and Germer in 1927.
Einstein received the Nobel Prize in 1921 for his work with the wave–particle duality on photons (especially explaining the photoelectric effect thereby), and de Broglie followed in 1929 for his extension to other particles.
Quantum electrodynamics
The quantum mechanical theory of light and electromagnetic radiation continued to evolve through the 1920s and 1930s, and culminated with the development during the 1940s of the theory of quantum electrodynamics, or QED.
This so-called quantum field theory is among the most comprehensive and experimentally successful theories ever formulated to explain a set of natural phenomena.
QED was developed primarily by physicists Richard Feynman, Freeman Dyson, Julian Schwinger, and Shin-Ichiro Tomonaga. Feynman, Schwinger, and Tomonaga shared the 1965 Nobel Prize in Physics for their contributions.
wikipedia
The wave theory was successful in explaining nearly all optical and electromagnetic phenomena, and was a great triumph of nineteenth century physics.
By the late nineteenth century, however, a handful of experimental anomalies remained that could not be explained by or were in direct conflict with the wave theory.
One of these anomalies involved a controversy over the speed of light. The constant speed of light predicted by Maxwell's equations and confirmed by the Michelson-Morley experiment contradicted the mechanical laws of motion that had been unchallenged since the time of Galileo, which stated that all speeds were relative to the speed of the observer.
In 1905, Albert Einstein resolved this paradox by proposing that space and time appeared to be changeable entities, which accounted for the constancy of the speed of light.
Einstein also proposed a previously unknown fundamental equivalence between energy and mass with his famous equation
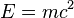
|
where E is energy, m is, depending on the context, the rest mass or the relativistic mass, and c is the speed of light in a vacuum.
Particle theory revisited
Another experimental anomaly was the photoelectric effect, by which light striking a metal surface ejected electrons from the surface, causing an electric current to flow across an applied voltage.
Experimental measurements demonstrated that the energy of individual ejected electrons was proportional to the frequency, rather than the intensity, of the light.
Furthermore, below a certain minimum frequency, which depended on the particular metal, no current would flow regardless of the intensity.
These observations appeared to contradict the wave theory, and for years physicists tried in vain to find an explanation.
In 1905, Einstein solved this puzzle as well, this time by resurrecting the particle theory of light to explain the observed effect. Because of the preponderance of evidence in favor of the wave theory, however, Einstein's ideas were met initially with great skepticism among established physicists. But eventually Einstein's explanation of the photoelectric effect would triumph, and it ultimately formed the basis for wave–particle duality and much of quantum mechanics.
Quantum theory
A third anomaly that arose in the late 19th century involved a contradiction between the wave theory of light and measurements of the electromagnetic spectrum emitted by thermal radiators, or so-called black bodies. Physicists struggled with this problem, which later became known as the ultraviolet catastrophe, unsuccessfully for many years.
In 1900, Max Planck developed a new theory of black-body radiation that explained the observed spectrum. Planck's theory was based on the idea that black bodies emit light (and other electromagnetic radiation) only as discrete bundles or packets of energy. These packets were called quanta, and the particle of light was given the name photon, to correspond with other particles being described around this time, such as the electron and proton.
A photon has an energy, E, proportional to its frequency, f, by where h is Planck's constant, λ is the wavelength and c is the speed of light. Likewise, the momentum p of a photon is also proportional to its frequency and inversely proportional to its wavelength: As it originally stood, this theory did not explain the simultaneous wave- and particle-like natures of light, though Planck would later work on theories that did. In 1918, Planck received the Nobel Prize in Physics for his part in the founding of quantum theory.
Wave–particle duality
The modern theory that explains the nature of light includes the notion of wave–particle duality, described by Albert Einstein in the early 1900s, based on his study of the photoelectric effect and Planck's results.
Einstein asserted that the energy of a photon is proportional to its frequency.
More generally, the theory states that everything has both a particle nature and a wave nature, and various experiments can be done to bring out one or the other.
The particle nature is more easily discerned if an object has a large mass, and it was not until a bold proposition by Louis de Broglie in 1924 that the scientific community realised that electrons also exhibited wave–particle duality. The wave nature of electrons was experimentally demonstrated by Davisson and Germer in 1927.
Einstein received the Nobel Prize in 1921 for his work with the wave–particle duality on photons (especially explaining the photoelectric effect thereby), and de Broglie followed in 1929 for his extension to other particles.
Quantum electrodynamics
The quantum mechanical theory of light and electromagnetic radiation continued to evolve through the 1920s and 1930s, and culminated with the development during the 1940s of the theory of quantum electrodynamics, or QED.
This so-called quantum field theory is among the most comprehensive and experimentally successful theories ever formulated to explain a set of natural phenomena.
QED was developed primarily by physicists Richard Feynman, Freeman Dyson, Julian Schwinger, and Shin-Ichiro Tomonaga. Feynman, Schwinger, and Tomonaga shared the 1965 Nobel Prize in Physics for their contributions.
wikipedia
No comments:
Post a Comment